Why does the capacity of lithium battery become lower in winter?
Since entering the market, lithium-ion batteries have been widely used because of their long life, large specific capacity and no memory effect. There are some problems such as low capacity, serious attenuation, poor cycle rate performance, obvious lithium precipitation and unbalanced lithium intercalation when lithium ion batteries are used at low temperature. However, with the continuous expansion of application fields, the constraints caused by the poor low-temperature performance of lithium-ion batteries become more and more obvious.
It is reported that the discharge capacity of lithium-ion batteries at -20℃ is only about 31.5% of that at room temperature. The working temperature of traditional lithium ion battery is between -20~+55℃. However, in aerospace, military industry, electric vehicles and other fields, the battery is required to work normally at -40℃. Therefore, it is of great significance to improve the low-temperature properties of lithium-ion batteries.
Factors restricting the low-temperature performance of lithium-ion batteries
At low temperature, the viscosity of electrolyte increases, even partially solidifies, which leads to the decrease of conductivity of lithium ion battery. The compatibility between electrolyte, negative electrode and diaphragm becomes worse in low temperature environment. At low temperature, lithium is seriously precipitated from the negative electrode of lithium-ion battery, and the precipitated metallic lithium reacts with electrolyte, and its product deposition leads to the increase of the thickness of solid electrolyte interface (SEI). At low temperature, the diffusion system in the active material of lithium-ion battery decreases, and the charge transfer impedance (Rct) increases significantly.
Discussion on factors affecting low temperature performance of lithium ion battery
Expert opinion 1: Electrolyte has the greatest influence on the low-temperature performance of lithium-ion batteries, and the composition and physicochemical properties of electrolyte have an important influence on the low-temperature performance of batteries. The problems that the battery faces when circulating at low temperature are: the viscosity of electrolyte will increase, and the ion conduction speed will slow down, resulting in the mismatch of electron migration speed in the external circuit, so the battery will be seriously polarized and the charge and discharge capacity will decrease sharply. Especially when charged at low temperature, lithium ions can easily form lithium dendrites on the surface of the negative electrode, leading to battery failure.
The low-temperature performance of the electrolyte is closely related to the conductivity of the electrolyte itself. With high conductivity, the electrolyte can transport ions quickly and can exert more capacity at low temperature. The more lithium salts dissociate in the electrolyte, the more migration numbers and the higher conductivity. The higher the conductivity, the faster the ion conduction rate, the smaller the polarization and the better the performance of the battery at low temperature. Therefore, high conductivity is a necessary condition to achieve good low-temperature performance of lithium-ion batteries.
The conductivity of electrolyte is related to the composition of electrolyte. Reducing the viscosity of solvent is one of the ways to improve the conductivity of electrolyte. The good fluidity of solvent at low temperature is the guarantee of ion transport, and the solid electrolyte film formed by electrolyte at the negative electrode at low temperature is also the key to affect lithium ion conduction, and RSEI is the main impedance of lithium ion battery at low temperature.
Expert 2: The main factor limiting the low-temperature performance of lithium-ion batteries is the rapidly increasing Li+ diffusion impedance at low temperature, not the SEI film.
Low temperature characteristics of cathode materials for lithium ion batteries
1. Low temperature characteristics of layered cathode materials.
Layered structure, which not only has the incomparable rate performance of one-dimensional lithium ion diffusion channel, but also has the structural stability of three-dimensional channel, is the earliest commercial cathode material for lithium ion batteries. Its representative substances are LiCoO2, Li(Co1-xNix)O2 and Li(Ni,Co,Mn)O2, etc.
Xie Xiaohua and others took LiCoO2/MCMB as the research object, and tested its low-temperature charge-discharge characteristics.
The results show that the discharge plateau decreases from 3.762V(0℃) to 3.207 V (–30℃) with the decrease of temperature. The total capacity of the battery also decreased sharply from 78.98 Ma h (0℃) to 68.55 Ma h (–30℃).
2. Low temperature characteristics of spinel cathode materials.
LiMn2O4 cathode material with spinel structure has the advantages of low cost and no toxicity because it contains no Co element.
However, the changeable valence state of Mn and Jahn-Teller effect of Mn3+ lead to structural instability and poor reversibility.
Peng Zhengshun and others pointed out that different preparation methods have great influence on the electrochemical properties of LiMn2O4 cathode materials. Taking Rct as an example, the Rct of LiMn2O4 synthesized by high-temperature solid-state method is obviously higher than that synthesized by sol-gel method, and this phenomenon is also reflected in the lithium ion diffusion coefficient. The main reason is that different synthesis methods have great influence on the crystallinity and morphology of the product.
3. Low temperature characteristics of phosphate cathode materials.
Because of its excellent volume stability and safety, LiFePO4, together with ternary materials, has become the main cathode material of power batteries at present. The poor low-temperature performance of Ferrous lithium phosphate is mainly due to the fact that its material itself is an insulator, with low electronic conductivity, poor lithium ion diffusivity and poor conductivity at low temperature, which increases the internal resistance of the battery, is greatly affected by polarization, and hinders the charging and discharging of the battery, so the low-temperature performance is not ideal.
Gu Yijie and others found that the coulombic efficiency of LiFePO4 decreased from 100% at 55℃ to 96% at 0℃ and 64% at -20℃ respectively. The discharge voltage decreased from 3.11V at 55℃ to 2.62V at -20℃.
Xing et al. modified LiFePO4 with nano-carbon, and found that the electrochemical performance of LiFePO4 was less sensitive to temperature and the low-temperature performance was improved after adding nano-carbon conductive agent. After modification, the discharge voltage of LiFePO4 decreased from 3.40V at 25℃ to 3.09V at -25℃, with a decrease of only 9.12%. And its battery efficiency is 57.3% at -25℃, which is higher than 53.4% without nano-carbon conductive agent.
Recently, LiMnPO4 has aroused great interest. It is found that LiMnPO4 has the advantages of high potential (4.1V), no pollution, low price and large specific capacity (170mAh/g). However, LiMnPO4 has lower ionic conductivity than LiFePO4, so in practice, Fe is often used to partially replace Mn to form LiMn0.8Fe0.2PO4 solid solution.
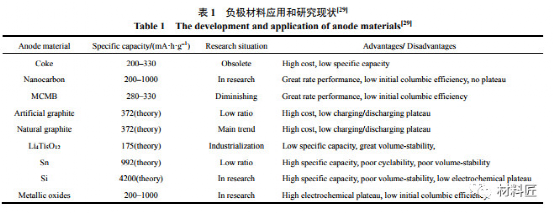
Low temperature characteristics of anode materials for lithium ion batteries
Compared with cathode materials, the low-temperature deterioration of cathode materials for lithium-ion batteries is more serious, mainly for the following three reasons:
When the battery is charged and discharged at low temperature and high rate, the polarization of the battery is serious, and a large amount of lithium metal is deposited on the surface of the negative electrode, and the reaction product of lithium metal and electrolyte is generally not conductive; From the thermodynamic point of view, the electrolyte contains a lot of C–O, C–N, etc.
Polar groups can react with negative electrode materials, and the formed SEI film is more susceptible to low temperature; It is difficult to embed lithium in carbon anode at low temperature, and there is asymmetry in charge and discharge.
Study on low temperature electrolyte
Electrolyte plays the role of transferring Li+ in lithium-ion batteries, and its ionic conductivity and SEI film-forming performance have significant effects on the low-temperature performance of the batteries. There are three main indexes to judge the quality of electrolyte for low temperature: ionic conductivity, electrochemical window and electrode reactivity. The level of these three indicators depends to a great extent on its constituent materials: solvent, electrolyte (lithium salt) and additives. Therefore, it is of great significance to study the low-temperature performance of each part of the electrolyte for understanding and improving the low-temperature performance of the battery.
Low temperature characteristics of EC-based electrolyte Compared with chain carbonate, cyclic carbonate has compact structure, large force and higher melting point and viscosity. However, due to the large polarity brought by the ring structure, it often has a large dielectric constant. The large dielectric constant, high ionic conductivity and excellent film-forming performance of EC solvent effectively prevent solvent molecules from co-inserting, which makes it indispensable. Therefore, most commonly used low-temperature electrolyte systems are based on EC, and then mixed with low-melting-point small molecular solvents. Lithium salt is an important component of electrolyte. Lithium salt in electrolyte can not only improve the ionic conductivity of the solution, but also reduce the diffusion distance of Li+ in the solution. Generally speaking, the greater the concentration of Li+ in the solution, the greater the ionic conductivity. However, the concentration of lithium ion in electrolyte is not linearly related to the concentration of lithium salt, but parabolic. This is because the concentration of lithium ion in the solvent depends on the dissociation and association of lithium salt in the solvent.
Study on low temperature electrolyte
In addition to the battery composition itself, the process factors in actual operation will also have a great impact on the battery performance.
(1) preparation process. Yaqub et al. studied the influence of electrode load and coating thickness on the low-temperature performance of LiNi0.6Co0.2Mn0.2O2 /Graphite battery, and found that the smaller the electrode load, the thinner the coating layer and the better its low-temperature performance in terms of capacity retention.
(2) Charge and discharge state. Petzl et al. studied the effect of low-temperature charging and discharging state on the cycle life of the battery, and found that when the discharge depth is large, it will cause greater capacity loss and reduce the cycle life.
(3) Other factors. The surface area, pore size, electrode density, wettability between electrode and electrolyte, and separator all affect the low-temperature performance of lithium-ion batteries. In addition, the influence of material and process defects on the low-temperature performance of the battery can not be ignored.
Summary
In order to ensure the low-temperature performance of lithium-ion batteries, the following points need to be done:
(1) forming a thin and dense SEI film;
(2) Ensure that Li+ has a large diffusion coefficient in active substances;
(3) The electrolyte has high ionic conductivity at low temperature.
In addition, we can find another way to look at another kind of lithium-ion battery-all-solid-state lithium-ion battery. Compared with conventional lithium-ion batteries, all-solid-state lithium-ion batteries, especially all-solid-state thin-film lithium-ion batteries, are expected to completely solve the capacity attenuation and cycle safety problems of batteries used at low temperatures.



